COVID-19 vaccine rollout starts in the US
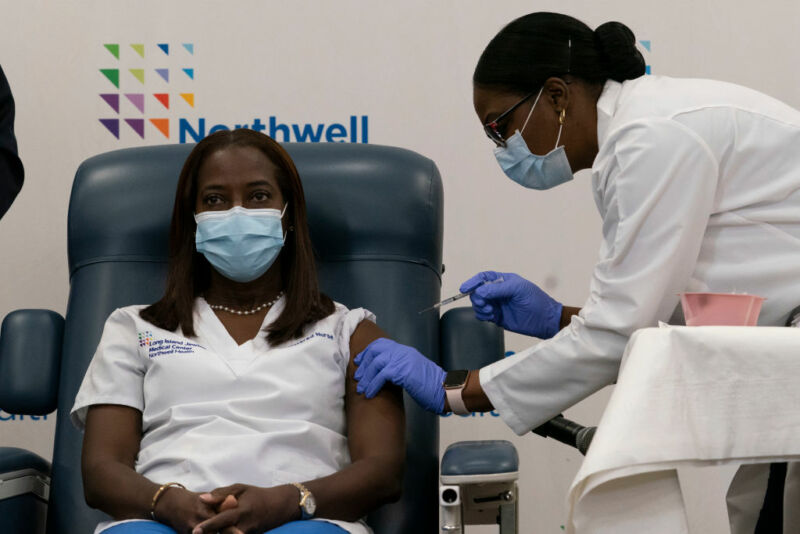
In the wake of positive results from large-scale trials, many countries are adopting the COVID-19 vaccine produced by the Pfizer/BioNTech collaboration. That led to some of the first vaccinations of the general population last week. In the US, it took until Friday for the vaccine to receive an Emergency Use Authorization from the Food and Drug Agency. After that decision, shipments of the vaccine started almost immediately, and reports are now coming in of the first vaccinations in the US.
The Pfizer/BioNTech vaccine uses a technology that, while not uncommon in biology labs, hasn’t been used in vaccinations before. The vaccine consists of RNA molecules with a fatty chemical coating. The coat will fuse with the surface of human cells, dumping the RNA inside them, where it directs the production of the coronavirus’s Spike protein. Once a person’s cells produce Spike, the immune system reacts to it and becomes primed to protect the person from infection by the actual virus.
Unfortunately, this formulation requires that the vaccine be kept at very low temperatures during shipping. In the US, both FedEx and UPS are providing trucks equipped to handle these requirements; American and United Airlines are also collaborating to get the vaccine to distribution centers.
For many months, however, the primary limitation on vaccine availability won’t be shipping but rather manufacturing capacity. While supplies are constrained, vaccines will be given out according to a combination of need and risk. For now, the only ones who should be getting doses are health care workers, who are at high risk of exposure, and the elderly, who experience the worst outcomes from COVID-19.
On Sunday, word leaked that the White House was planning to provide its staff early access. While the staff is at high risk of exposure due to the administration’s inability to follow public health guidance, many of them are already immune due to previous exposure, and others don’t fall into an obvious high-risk category. Shortly after reports of the plan became public, however, President Trump announced that the plan would be put on hold.
As a result, one of the earliest reports of a person receiving the vaccine was someone who should be getting it: a health care worker in Queens, New York, named Sandra Lindsay. Speaking afterward, Lindsay made it clear that she was aware that the vaccine would be in short supply for months and that the politicization of the pandemic made some hesitant about receiving it. So she used her time in the spotlight to encourage vaccination and drive home the importance of following public health advice until that’s an option for everyone.
We’re in a pandemic, and so we all need to do our part to put an end to the pandemic and to not give up so soon. There’s light at the end of the tunnel, but we still need to continue to wear our mask, to social distance. I believe in science. As a nurse, my practice is guided by science, and so I trust that. What I don’t trust is that if I contract COVID—I don’t know how it will impact me or those who I come in contact with. So I encourage everyone to take the vaccine.
Given the current prevalence of the virus in the US, widespread vaccination will not come quickly enough to change the dynamics of the pandemic this winter. As a result, the nation’s final death toll from the COVID-19 pandemic will be heavily influenced by our ability to follow public health advice on mask use, social distancing, and other responsible behaviors for the next several months. We’re fortunate to have Lindsay take her opportunity to drive home this message.
https://arstechnica.com/?p=1729714