Scientists recreated classic origin-of-life experiment and made a new discovery

In 1952, a University of Chicago chemist named Stanley Miller and his adviser, Harold Urey, conducted a famous experiment. Their results, published the following year, provided the first evidence that the complex organic molecules necessary for the emergence of life (abiogenesis) could be formed using simpler inorganic precursors, essentially founding the field of prebiotic chemistry. Now a team of Spanish and Italian scientists has recreated that seminal experiment and discovered a contributing factor that Miller and Urey missed. According to a new paper published in the journal Scientific Reports, minerals in the borosilicate glass used to make the tubes and flasks for the experiment speed up the rate at which organic molecules form.
In 1924 and 1929, respectively, Alexander Oparin and J.B.S. Haldane had hypothesized that the conditions on our primitive Earth would have favored the kind of chemical reactions that could synthesize complex organic molecules from simple inorganic precursors—sometimes known as the “primordial soup” hypothesis. Amino acids formed first, becoming the building blocks that, when combined, made more complex polymers.
Miller set up an apparatus to test that hypothesis by simulating what scientists at the time believed Earth’s original atmosphere might have been. He sealed methane, ammonia, and hydrogen inside a sterile 5-liter borosilicate glass flask, connected to a second 500-ml flask half-filled with water. Then Miller heated the water, producing vapor, which in turn passed into the larger flask filled with chemicals, creating a mini-primordial atmosphere. There were also continuous electric sparks firing between two electrodes to simulate lighting. Then the “atmosphere” was cooled down, causing the vapor to condense back into water. The water trickled down into a trap at the bottom of the apparatus.
That solution turned pink after one day and deep red after a week. At that point, Miller removed the boiling flask and added barium hydroxide and sulfuric acid to stop the reaction. After evaporating the solution to remove any impurities, Miller tested what remained via paper chromatography. All known life consists of just 20 amino acids. Miller’s experiment produced five amino acids, although he was less certain about the results for two of them.
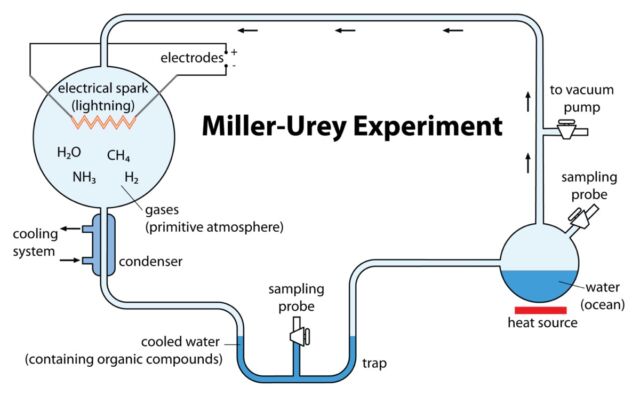
When Miller showed his results to Urey, the latter suggested a paper should be published as soon as possible. (Urey was senior but generously declined to be listed as co-author, lest this lead to Miller getting little to no credit for the work.) The paper appeared in 1953 in the journal Science. “Just turning on the spark in a basic pre-biotic experiment will yield 11 out of 20 amino acids,” Miller said in a 1996 interview. The original apparatus has been on display at the Denver Museum of Nature and Science since 2013.
Miller died in 2007. Shortly before he passed, one of his students, Jeffrey Bada, now at the University of San Diego, inherited all his mentor’s original equipment. This included several boxes filled with vials of dried residues from the original experiment. Those 1952 samples were re-analyzed the following year using the latest chromatography methods, revealing that the original experiment actually produced even more compounds (25) than had been reported at the time.
Miller had also performed additional experiments simulating conditions similar to those of a water-vapor-rich volcanic eruption, which involved spraying steam from a nozzle at the spark discharge. Bada and several colleagues re-analyzed the original samples from those experiments, too, and found this environment produced 22 amino acids, five amines, and several hydroxylated molecules. So the original experiments were even more successful than Miller and Urey realized.
There have been many, many more experiments on abiogenesis over the ensuing decades, but co-author Joaquin Criado-Reyes of the Universidad de Granada in Spain and his collaborators thought that one potential factor had been overlooked: the role of the borosilicate glass that comprised the flasks and tubes Miller had used. They noted that Miller’s simulated atmosphere was highly alkaline, which should cause the silica to dissolve. “Therefore, it could be expected that upon contact of the alkaline water with the inner wall of the borosilicate flask, even this reinforced glass will slightly dissolve, releasing silica and traces of other metal oxides [into the vapor],” the authors wrote.
https://arstechnica.com/?p=1807727