
An independent committee of experts that advises the Centers for Disease Control and Prevention voted Thursday to recommend booster doses of the Pfizer/BioNTech mRNA vaccine for persons ages 65 and older; residents of long-term care facilities ages 18 and up; and those ages 50 to 64, who have underlying medical conditions that put them at high risk.
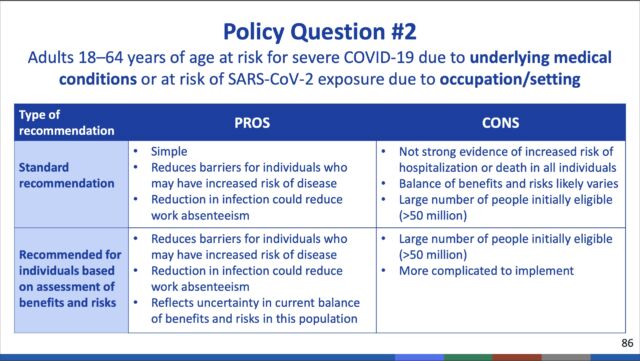
Additionally, the committee recommended that people ages 18 to 49 with such underlying medical conditions should have the option to receive a booster dose after weighing their individual benefits and risks.
The Pfizer/BioNTech boosters are recommended to be given at least six months after the initial two doses. And they are intended only for people who received an initial series of two Pfizer/BioNTech vaccines—not those who received Moderna or Johnson & Johnson vaccines.
The recommendations by the committee—the Advisory Committee on Immunization Practices, or ACIP—now head to CDC director Rochelle Walensky for a sign-off before becoming official federal policy.
Down vote
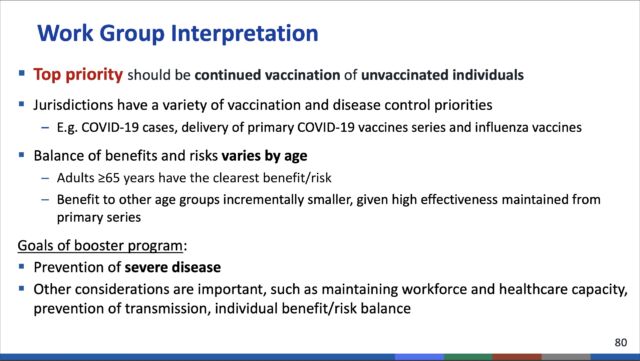
Notably, the ACIP committee voted against recommending booster doses for those ages 18 and older at high risk based on occupational or institutional exposures to the pandemic coronavirus. This category would have included health care workers, front-line workers, teachers, day care providers, grocery store workers, and people in prisons and homeless shelters—among others.
That expansive category of people is indeed included in language from the Food and Drug Administration, which issued an emergency authorization for boosters late Wednesday. That authorization comes as an amendment to the Pfizer/BioNTech vaccine’s Emergency Use Authorization. And it permitted the boosters to go to individuals whose “frequent institutional or occupational exposure to SARS-CoV-2 puts them at high risk of serious complications of COVID-19 including severe COVID-19.”
But in an extensive debate today, the CDC committee ultimately voted that the group was simply too large and that such a recommendation would essentially result in a booster free-for-all. Additionally, such a mass booster recommendation might send the message that vaccine efficacy is failing—when, in fact, it is not. Throughout a two-day-long meeting, the committee reviewed data showing that the initial two-dose Pfizer vaccine continues to provide strong protection against severe disease and death in those under age 65.
Still, the decision was not easy. Advisors shared personal stories of health care providers who are overwhelmed by surges of COVID-19 patients and could benefit from any bit of extra protection. A booster, some argued, would help keep them healthy and able to keep working. Others spoke of treating children too young to be vaccinated who contracted severe cases of COVID-19. Boosting the adults around those children may have spared them from that disease, others said.
But, on a population-wide scale, a narrow majority of the committee felt the data did not warrant additional boosts for those not at direct risk. In a 9-6 vote, the committee voted against boosters for people with occupational or institutional risks.
Interim recommendations
The committee voted unanimously, however, that those 65 and older and those living in long-term care facilities should get boosters. Data does suggest that these groups are at increased risk of severe disease and death—as are people with underlying medical conditions.
-
Vaccine efficacy estimates
-
People ages 65 and up will benefit most from boosters
-
More benefits to boosters in older age groups
-
Takeaways for those 65 and up
-
Rationale for people in long-term care facilities
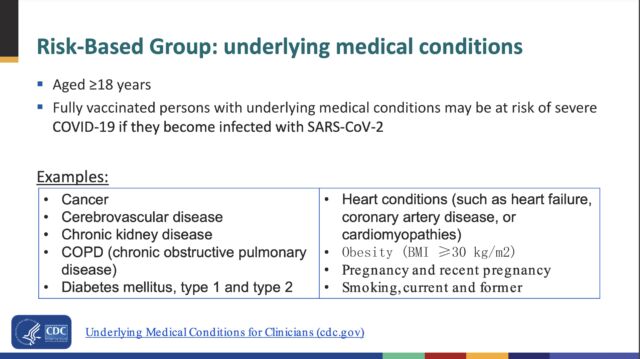
CDC experts in the meeting did not provide a complete list of underlying medical conditions that would warrant a booster dose. But they did offer a partial list that included: cancer, cerebrovascular disease, chronic kidney disease, COPD (chronic obstructive pulmonary disease), diabetes mellitus (type 1 and type 2), heart conditions (such as heart failure, coronary artery disease, or cardiomyopathies), obesity (BMI ≥30 kg/m2), pregnancy and recent pregnancy, and smoking (current and former).
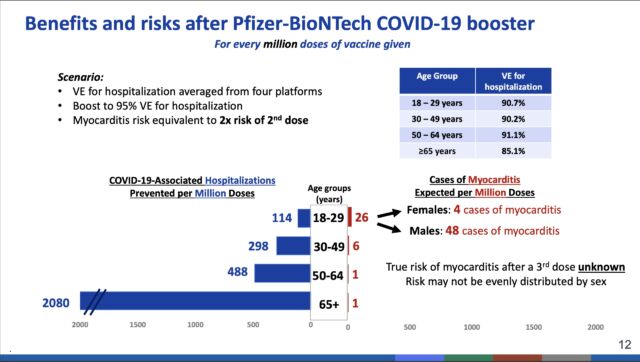
For those with these conditions in the 18 to 49 age range, the committee refrained from issuing a full-blown recommendation to get a booster dose. Instead, the researchers supported a more flexible option, in which a booster dose is recommended after the benefits and risks are weighed for each individual. Those benefit and risk assessments can get hairy for younger groups, particularly young males ages 18 to 29. This group is at higher relative risk of developing inflammation of the heart (myocarditis) as a side effect of vaccination. The risk is small, but—potentially—so are the benefits from a third vaccine dose in an age group that is at relatively low risk of severe outcomes even when unvaccinated.
Though ACIP members agonized today over who should and who shouldn’t get a booster dose, the ACIP also lamented the limited benefit any boosters will provide. ACIP voting member Helen “Keipp” Talbot, an infectious disease expert at Vanderbilt University, spoke of hospitals crammed with unvaccinated patients sick with COVID-19. “In all honesty, we could give boosters to people, but that’s not really the answer to this pandemic,” Dr. Talbott said. “I feel like we’re putting lipstick on frogs. This is not going to solve the pandemic.”
The committee’s recommendations now move to CDC director Walensky’s desk. If the recommendations are adopted (which they likely will be), vaccine providers will be required to follow them. That’s because the vaccines are paid for and distributed by the federal government, which required providers to adhere to the FDA’s EUA language and ACIP recommendations.
However, as CDC officials noted several times throughout today’s meeting, the current booster recommendations are merely “interim” recommendations. ACIP and US officials expect that more boosters and new recommendations will come out in the coming weeks.
https://arstechnica.com/?p=1797975

