Quasicrystals are a unique class of materials with considerable promise for practical applications because of their unusual properties. But progress toward realizing that commercial potential has been hampered by the fact that the usual manufacturing processes for quasicrystals are prone to producing defects in the form of tiny cracks between crystals known as grain boundaries. A new paper published in the journal Nature Communications found that under certain conditions, quasicrystals can heal themselves—potentially reviving commercial interest in these materials.
The earliest quasicrystals found were metal alloys, usually aluminum with one or more other metals. That has made them useful for a handful of practical applications, such as non-stick coatings for frying pans and anti-corrosive coatings for surgical equipment. But scientists would love to create more complex quasicrystals that are capable, for example, of manipulating light to create new kinds of camouflage or cloaking.
“One reason why industry gave up on quasicrystals is because they’re full of defects,” said co-author Ashwin Shahani, a materials scientist at the University of Michigan. “But we’re hoping to bring quasicrystals back into the mainstream. And this work hints that it can be done.”
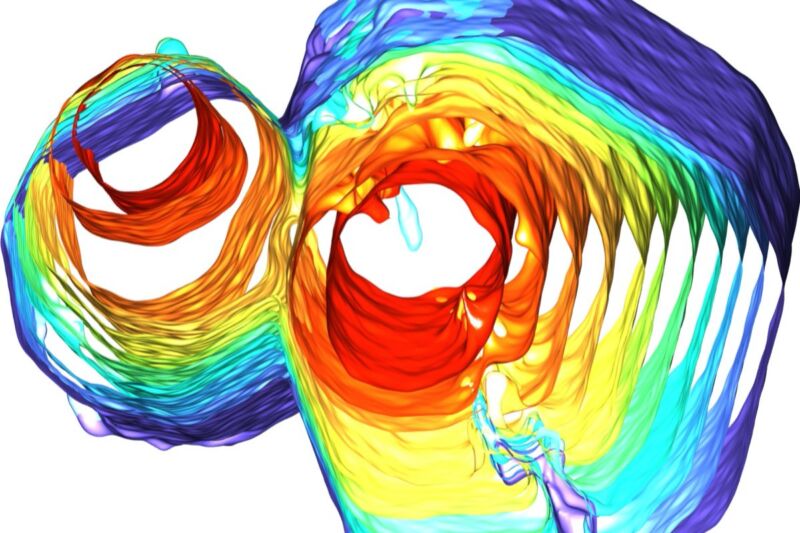
As I’ve written previously, the very definition of a crystal assumes a precisely symmetrical ordering of atoms in periodic patterns that repeat over and over in a 3D lattice. The patterns look the same no matter which direction you look at them, but quasicrystals are different. They clearly follow mathematical rules, but each cell has a slightly different configuration of cells nearby rather than repeating in an identical pattern. It’s that unique structure that gives quasicrystals their unusual properties.
Think about tiling a bathroom floor. The tiles can only be in certain symmetrical shapes (triangles, squares, or hexagons); otherwise, you wouldn’t be able to fit them together without leaving gaps or overlapping tiles. Pentagons, icosahedrons, and similar shapes with different symmetries that never precisely repeat just won’t work—except in the case of quasicrystals, where nature decided they could work. The trick is to fill the gaps with other kinds of atomic shapes to create the unlikely aperiodic structure.
An Israeli physicist named Daniel Shechtman won the 2011 Nobel Prize in Chemistry for his discovery of quasicrystals in 1982 in rapidly quenched aluminum alloys. Princeton physicist Paul Steinhart discovered the first known naturally occurring quasicrystals in 2008. In 2018, chemists at Brown University created a new kind of self-assembling quasicrystal out of a single type of nanoparticle: a tetrahedral (pyramid-shaped) quantum dot.
These nanoparticles are also anisotropic, i.e., they have different properties depending upon their orientation relative to each other. When placed on a liquid surface, they assembled into 10-sided structures called decagons, and these decagons in turn stitched themselves together to form a quasicrystal lattice with tenfold symmetry. What makes this work is the flexible edges of the decagons, which can flatten out at key points to morph into polygons with five, six, seven, eight, or nine sides—whatever is needed to fill the inevitable gaps between decagons to make the quasicrystal.
Earlier this year, University of Utah scientists demonstrated that ultrasound waves can be used to organize carbon nanoparticles in water into the same aperiodic pattern found in quasicrystals. And in May, Steinhardt and several colleagues announced the discovery of a previously unknown quasicrystal in red trinitite from the first detonation of an atomic bomb. Even better, the researchers could determine exactly where and how the quasicrystal formed thanks to historical records from the Manhattan Project.
https://arstechnica.com/?p=1803153

