There’s a strong consensus that life on Earth got its start through RNA, a close chemical sibling of DNA. Over the last few decades, researchers have described how individual RNA bases can spontaneously polymerize, forming longer chains that could ultimately catalyze key chemical reactions, including building even longer RNA molecules. As a result, it’s clear that RNA can perform two functions: carrying heritable genetic information just as DNA does and carrying out the instructions encoded by that information.
There’s far less agreement, however, on how those RNA bases themselves first form. These bases have a combination of one of two types of flat, ringed structures linked to a small, ring-shaped sugar. Over time, researchers have found sets of chemical reactions that could start with simple chemicals likely to be found on the early Earth and end up with one of the three more complex chemicals needed to form RNA. But the conditions needed for these reactions weren’t compatible, raising questions about how an RNA molecule could ever form from these reactions.
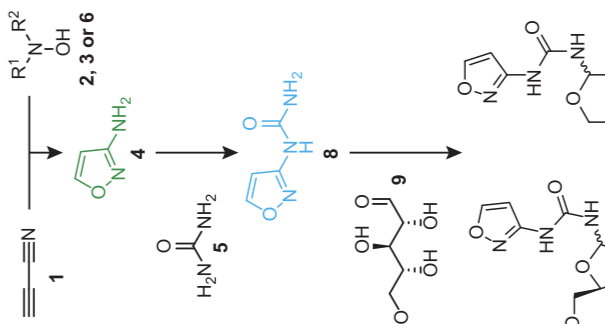
Now, a group of chemists has figured out a way to form the portions of RNA that give it its identity starting from a simple set of chemicals. The work relies on materials that can easily be provided by a volcanic environment. And driving the reactions forward requires little more than a few wet/dry cycles.
Starting from scratch
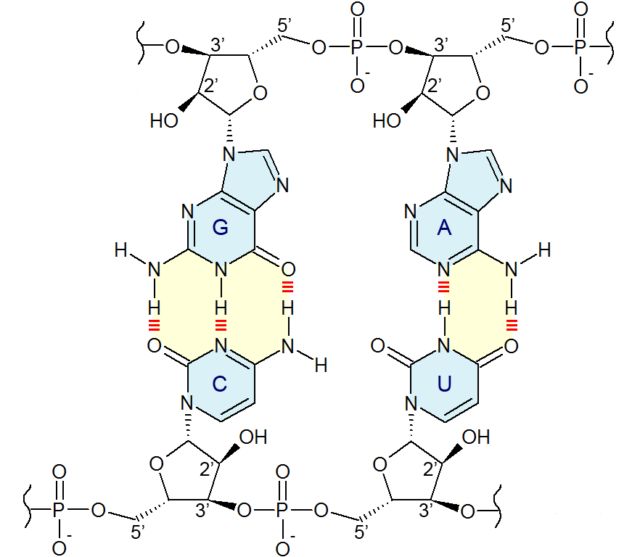
Both DNA and RNA can form a double helix, with the exterior of the helix formed by sugars linked through phosphates. It’s the chemicals linked to these sugars, called bases, that provide a stretch of double helix with information content, through the changing order of the four bases: A, T, C, and G in DNA and A, U, C, and G in RNA. These bases can be divided into two categories: two-ringed structures (A and G) and single-ringed structures (T, U, and C). The base pairing that holds the double helix together always involves pairing a one-ringed base with a two-ringed base, which maintains a constant width of the helix.
Starting from simple chemicals that were likely to be present on the early Earth, researchers have proposed pathways that can synthesize both types of bases, with rings being formed by a reaction that chemically links a precursor to the sugar. But the conditions in which these sets of reactions work are very different, meaning that they can’t be formed at the same time. Which, if you expect them to end up in the same molecule, is pretty limiting.
So the team of researchers behind the new work decided to see if they could find conditions under which both types of base could form.
As with other work on origin-of-life issues, the researchers tried to follow a set of general principles. The starting chemicals had to be simple and likely to be present on the early Earth. One key starting material, for example, has already been spotted in interstellar clouds. Others, like nitrite and sulfite, are known to be released in gases at volcanic vents.
In addition, all the reactions should take place without the need for any exotic materials. Two of the steps the researchers describe do require metals, but the metals (iron and zinc) are both very common in the Earth’s crust. Finally, the reactions should be robust, in that the yields should be reasonably high, and they shouldn’t be very sensitive to initial conditions such as temperature.
While the team undoubtedly tried a variety of methods that turned out to be dead ends, the reaction series they came up with relied on a critical step: letting things dry out. There are a lot of contexts in which environments alternate between wet and dry: rain vs. drought, high vs. low tides, and so on. These changes can have a dramatic effect on chemical reactions. They can determine which chemicals are washed away and which get left behind to react further.
But it can also drive some chemical reactions forward. Many reactions in organic chemistry involve combining chemicals in a way that oxygen and hydrogen atoms are removed, liberating a water molecule as a result. As a reaction mixture dries out, these reactions become increasingly favorable, which can promote the formation of chemicals that wouldn’t form in a wet environment.
In a final twist, it turns out that a key ingredient in the formation of one-ringed bases was a liquid with a boiling point more than twice that of water’s. As the reaction dried out, the liquid remained liquid and dissolved key chemicals in the formation of two-ringed bases, allowing further reactions to proceed.
In the end, the authors tested a series of separate reactions that produced each of the four bases from an identical set of starting materials but requiring different intermediates for each reaction (things like iron, a specific mineral, and so on). Then, satisfied that it worked, the researchers put all the intermediates in a single pot and were able to show that the final mixture contained all four bases. That’s the first time this has ever been demonstrated.
That said, it’s not a complete solution, as the final reaction involves a sugar that has to be provided separately. While there are known ways of making sugars from equally simple starting materials, those methods require conditions that aren’t compatible with these reactions. So we still can’t make an entire RNA molecule starting with simple conditions.
The conditions also aren’t entirely simple, as there are a number of reaction intermediates that must be supplied. In addition to the iron, zinc, and a mineral called lüneburgite, there are things like urea and a source of sulfur-hydrogen bonds. While it’s plausible that all of these things were available on the early Earth, there will undoubtedly be some discussion about whether they were present in the same place and under the requisite conditions. And there’s the issue of the fact that the sugar needs to be provided separately.
All of which is another way of saying that this doesn’t completely solve the question of how life could arise from simple precursors. But that doesn’t take away from the authors’ accomplishment: “We show that the key building blocks of life can be created without the need for sophisticated isolation and purification procedures of reaction intermediates that are common in traditional organic chemistry.”
Science, 2019. DOI: 10.1126/science.aax2747 (About DOIs).
https://arstechnica.com/?p=1579873

