Life performs many astonishing feats of chemistry, building complex molecules that can take us years to figure out how to synthesize. And the thermodynamics of these reactions are often fascinating—in many cases, life lives on the edge, at risk of seeing critical reactions bog down and run in reverse.
Now, some researchers have figured out a way to force bacteria to run a chemical reaction in reverse. Rather than breaking down a simple molecule into carbon dioxide, the bacteria will ingest carbon dioxide and spit out formic acid, a chemical that already has lots of uses—and could be used as fuel or to sequester carbon. The secret? Force-feed the bacteria the raw ingredients for the chemical reaction.
Enzymes and catalysis
The proteins that act as enzymes are nothing more than catalysts. The complex three-dimensional shapes of these proteins stabilize intermediate states of chemical reactions, lowering the energy required to reach them. This essentially lowers the energetic hill that has to be climbed to get between a set of reactants and a set of products. But if the overall energy of the reactants and products isn’t very different, then that smaller hill will also let things run in the opposite direction: the enzyme will happily form a reaction intermediate from the product and spit out the original reactants.
Life has all sorts of ways of avoiding this. In many cases, the reactants end up being used rapidly, keeping them from lingering about and running into the enzyme again. In others, an energy-carrying molecule gets used to force the reaction to only run in a single direction. But there are plenty of cases where a build-up of the products can take place, causing the enzyme to idle, catalyzing forward and reverse reactions at equal rates. Purified enzymes, given a large supply of the products, can run the reaction in reverse, producing lots of the normal reactants.
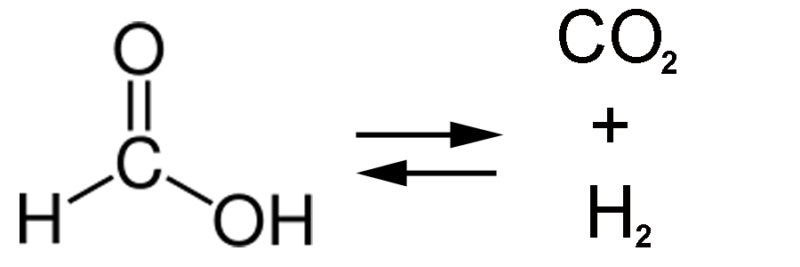
That’s the intellectual backdrop for the new work. The researchers were presumably looking for an enzyme-catalyzed reaction that normally produces some carbon dioxide as a product. There are a lot of these, but the authors then filtered the list down by looking for those reactions where the energy difference between the reactants and products is small. One of these is the digestion of formic acid, the small molecule shown above. Formic acid is essentially carbon dioxide with two hydrogens attached, and E. coli has an enzyme that starts with formic acid and spits out CO2 and H2. In this case, the energy difference between formic acid and CO2 is about the same as the energy content of H2, so the reaction could run in reverse, converting CO2 into formic acid.
Running in reverse
But running the reaction in reverse would require lots of the end products, hydrogen and carbon dioxide. To do that, the authors reasoned, simply requires a bit of pressure. Putting a gas at pressure into water increases the rate at which it dissolves. Since these two gases are very small molecules, they should diffuse readily from a watery solution into cells, where they can reach the enzyme.
So the authors set up a bioreactor and put some E. coli into it. They then sealed the reactor and pumped the gases in at high pressure. Formic acid started to appear in the liquid the bacteria were in—apparently they eject it from the cell—at rates proportional to the pressure. So, everything they reasoned out in principle appeared to have worked.
From there on out, it was largely a matter of optimizing the yield. One of the problems is that formic acid is, as its name implies, acidic, and levels could eventually reach the point where they’d damage the bacteria. So the bioreactor was modified to read the pH of the reaction and inject chemicals that could balance the acidity. E. coli also has two other pathways that can digest formic acid, so the team knocked out key genes in those pathways, which increased the yield.
By the time they were done, the yield was over 100 percent—not only was the enzyme converting all the carbon dioxide that the researchers were feeding it, it was apparently scavenging some from other cellular processes and converting that as well.
So it’s clear we can do this. Do we want to?
Obviously, supplying hydrogen is the big issue with this reaction. But formic acid has already been given consideration as a hydrogen storage mechanism and can be made to work in fuel cells. So if production of hydrogen from spare renewable energy ever becomes economical, formic acid has a lot of things going for it. It’s a liquid at room temperature, and while it will burn, the probability of it burning is somewhat less than that of gasoline. While this won’t remove carbon dioxide from the atmosphere, it will at least avoid putting more into it.
We’ve also engineered a different strain of E. coli to grow using formic acid as a carbon source. So it’s possible to combine these two bits of engineering and force the cells to ingest carbon dioxide and hydrogen in order to survive. This should allow evolution to optimize this enzyme system to work in reverse. But it also opens up the door to using carbon dioxide to feed bacteria that produce useful chemicals, including drugs, more complex biofuels, and the raw materials for plastics. Plastics in particular might go some way toward starting to remove some of the carbon we’ve been putting into the atmosphere.
Current Biology, 2017. DOI: 10.1016/j.cub.2017.11.050 (About DOIs).
https://arstechnica.com/?p=1237779

