
People have been brewing beer for millennia, and the basic chemistry of fermentation is well understood. But thanks to advanced analytical techniques, scientists continue to learn more about the many different chemical compounds that contribute to the flavor and aroma of different kinds of beer. The latest such analysis comes courtesy of a team of German scientists who analyzed over 400 commercial beers from 40 countries. The scientists identified at least 7,700 different chemical formulas and tens of thousands of unique molecules, according to a recent paper published in the journal Frontiers in Chemistry. And they did it with a new approach that can analyze a sample in just 10 minutes.
“Beer is an example of enormous chemical complexity,” said co-author Philippe Schmitt-Kopplin of the Technical University of Munich and the Helmholtz Center in Munich. “And thanks to recent improvements in analytical chemistry, comparable in power to the ongoing revolution in the technology of video displays with ever-increasing resolution, we can reveal this complexity in unprecedented detail. Today it’s easy to trace tiny variations in chemistry throughout the food production process, to safeguard quality or to detect hidden adulterations.”
As I’ve written previously, all beer contains hops, a key flavoring agent that also imparts useful antimicrobial properties. To make beer, brewers mash and steep grain in hot water, which converts all that starch into sugars. This is traditionally the stage when hops are added to the liquid extract (wort) and boiled. That turns some of the resins (alpha acids) in the hops into iso-alpha acids, producing beer’s hint of bitterness. Yeast is then added to trigger fermentation, turning the sugars into alcohol. Some craft brewers prefer dry-hopping—hops are added during or after the fermentation stage, after the wort has cooled. They do this as a way to enhance the hoppy flavors without getting excessive bitterness, since there is no isomerization of the alpha acids.
A number of studies in recent years have investigated different chemical aspects of beers. For instance, a 2019 study found that the hoppy flavor of late-hopped beers is largely due to a compound called (3R)-linalool, which imparts citrus and floral notes. Other common aromatic compounds include myrcene (which smells like geraniums) and rose-scented geraniol. By far the most potent odorant in hops goes by the complicated moniker 4-mercapto-4-methylpentan-2-one (4MMP for short); it’s what gives certain craft beers that distinctive black currant aroma.
To help brewers better understand how sour beers develop their distinctive complex flavors, chemists at the University of Redlands in California have been tracking various chemical compounds that contribute to those flavor profiles, monitoring how their concentrations change over time during the aging process. Using NMR spectroscopy, they have studied the levels of acetic acid, lactic acid, and succinic acid, all of which are produced as the yeast ferments and contribute to the distinctive flavor profile of a sour beer. The chemists have also used liquid chromatography and time-of-flight mass spectrometry to identify and track changes in trace compounds that can also contribute to the overall flavor profile, such as phenolics or vanillin.
And earlier this year, we reported on the work of German scientists who devised an automated, efficient method for measuring and tracking trace aroma compounds called thiols (or mercaptans). These compounds include the aforementioned 4MMP as well as 3-mercapto-1-hexanol (3MH) and 3-mercaptohexyl acetate (3MHA), which impart grapefruit and passion fruit/guava aromas, respectively.
This latest study by Schmitt-Kopplin and colleagues at the Technical University of Munich focuses on the influence of different starch sources on the metabolic signatures of a broad range of beers. German brewers are subject to Purity Law, which dates back to 1516 (although it has been amended in later centuries). That means they can’t use anything in their beers except malt, hops, water, and yeast.
But many other beers today are produced via several different brewing processes and raw materials. There are wheat beers as well as beers produced from other malted grains like corn and rice. Rice is key to brewing Indian rice beers (zutho) and gluten-free beers, for instance, with brewers adding caramelized rice malt to the latter to get a richer aroma and amber color.
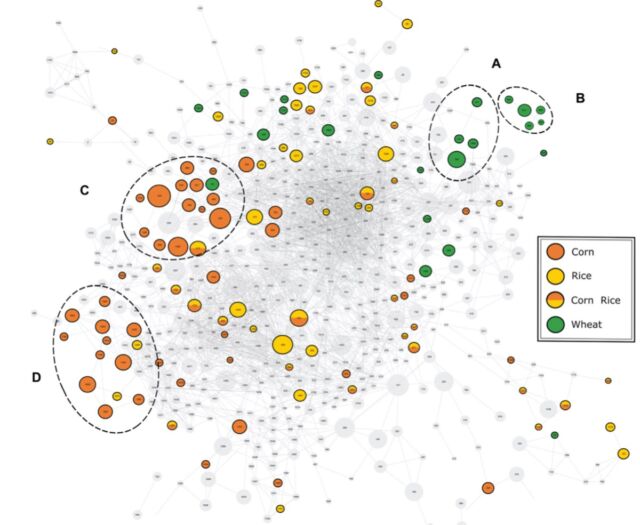
For their analysis, Schmitt-Kopplin et al. subjected 400 samples of beer—purchased from local grocery stores—brewed from all over the world (the US, Latin America, Europe, Africa, and East Asia) to two complementary mass spectrometry techniques. They used the first method to determine the beers’ chemical diversity and to predict chemical formulas for the metabolite ions in those beers. They used the second technique to figure out the exact molecular structure in a subsample of 100 beers. They were also able to reconstruct a full metabolic network of the complex reactions taking place during the brewing process.
The results: the team identified over 7,700 chemical formulas, each with as many as 25 different molecular structures. So any given beer will have tens of thousands of unique molecules contributing to its distinctive flavor, aroma, and other desirable qualities. It’s the deepest look yet at the impressive chemical diversity of popular beer styles, ranging from lagers and craft beers to abbey beers and geuzes (whether brewed from barley alone, or a combination of barley with wheat, rice, and corn). Furthermore, the team was able to differentiate between beers brewed with wheat, corn, and rice versus those brewed with barley alone.
“We show that this diversity originates in the variety of raw materials, processing, and fermentation,” said co-author Stefan Pieczonka, a graduate student at the Technical University of Munich. “The molecular complexity is then amplified by the so-called ‘Maillard reaction’ between amino acids and sugars which also gives bread, meat steaks, and toasted marshmallow their ‘roasty’ flavor. This complex reaction network is an exciting focus of our research, given its importance for food quality, flavor, and also the development of novel bioactive molecules of interest for health.”
DOI: Frontiers in Chemistry, 2021. 10.3389/fchem.2021.715372 (About DOIs).
https://arstechnica.com/?p=1787854

