An mRNA-based flu vaccine designed to offer long-lasting protection against a broad range of influenza viruses is now in a phase I clinical trial, the National Institutes of Health announced this week.
The trial brings the remarkable success of the mRNA vaccine platform to the long-standing efforts to develop a universal flu vaccine. Currently, health systems around the globe battle the seasonal scourge with shots that have to be reformulated each year to match circulating strains. This reformulation happens months before typical transmission, providing manufacturers time to produce doses at scale but also giving the strain circulation chances to shift unexpectedly. If the year’s shot is a poor match for the strains that circulate in a given season, efficacy against infection can be abysmal. Still, even when the shot is well-matched, people will need another shot next year.
“A universal influenza vaccine would be a major public health achievement and could eliminate the need for both annual development of seasonal influenza vaccines, as well as the need for patients to get a flu shot each year,” Hugh Auchincloss, acting director of the NIH’s National Institute of Allergy and Infectious Diseases, said in a news release. “Moreover, some strains of influenza virus have significant pandemic potential. A universal flu vaccine could serve as an important line of defense against the spread of a future flu pandemic.”
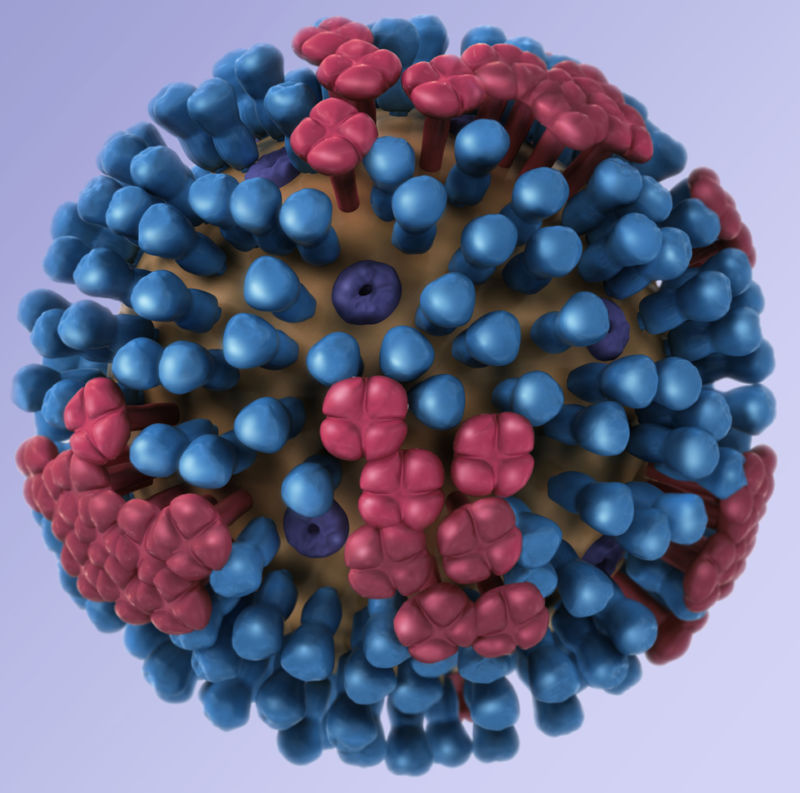
A successful design has been elusive. Flu vaccines often generate immune responses to fast-evolving bits of proteins on the outside of flu virus particles, hemagglutinin (Ha or H) and neuraminidase (Na or N). These proteins are responsible for helping the virus break into and out of human cells, respectively, during an infection. Both proteins look a little like lollipops stuck to the outside of the virus particle, with their tops evolving and being prime targets for potent antibodies against the virus.
For the universal vaccine design, NIH researchers have targeted not the tops of these proteins but a portion of the Ha protein’s stem—a highly conserved part of the protein that doesn’t evolve as quickly. Human antibodies that target this conserved region will likely target Ha proteins from a range of different flu strains in the same class. And, because this section doesn’t evolve as quickly, the vaccine could induce long-term immunity. With this design, the mRNA-based vaccine will include a snippet of genetic code in the form of mRNA that gives human cells the blueprints for this conserved stem region. From there, the immune system can learn to target it.
There’s already data to suggest this target could work. Before NIH researchers used an mRNA-based design, they developed a similar HA-stem targeting vaccine that appeared safe and effective in a phase I trial. The vaccine uses stabilized protein fragments of the Ha stem stuck to a nanoparticle. Last month, NIH researchers published results showing that this nanoparticle vaccine induced cross-reacting neutralizing antibodies against influenza viruses in the same virus group (H1). And those neutralizing antibodies stuck around for more than a year after vaccination. The vaccine candidate has advanced to a second trial. The researchers are hopeful that having multiple platforms in the works will increase their chances of getting a successful vaccine.
For now, the mRNA-based vaccine is starting with a small trial of just 50 people recruited through partners at Duke University. Three groups of 10 volunteers will get different vaccine doses to find the optimal dose. Once that’s found, 10 more will be vaccinated, and their responses will be compared to a 10-person control group that will be given a standard annual flu vaccine.
https://arstechnica.com/?p=1939739

