Today’s Nobel Prize in Medicine was awarded to three researchers who helped figure out how the human body senses oxygen. William Kaelin of Harvard Medical School, Sir Peter Ratcliffe of Oxford, and Gregg Semenza of Johns Hopkins have each been awarded an equal share of the prize for work that spanned decades.
But the work itself shows how it’s nearly impossible to study a complex pathway like this without relying on information from dozens of other researchers. And the details of how the pathway was teased apart read like a textbook of the methods of modern biology.
Who needs oxygen?
It might seem odd that we need an elaborate system to figure out how much oxygen we’re getting. After all, oxygen is central to our metabolism; if we run short, it should be obvious, right?
As with most things in biology, the facts are nowhere near that simple. Oxygen deprivation means something very different in a neuron than it does in a muscle cell or a liver cell. And there are distinct long- and short-term responses to oxygen levels. Run short during a workout and you’ll feel the burn; face a chronic shortage at altitude, and your body will undergo a series of physiological changes to adapt.
The latter aspect is what provided an entry to the current research. Other researchers had identified the hormone erythropoietin (the EPO made famous by many athletic doping scandals), which stimulates the production of red blood cells. The hormone is made by the kidneys and becomes elevated when people live at altitude, since the additional blood cells allow the body to use the limited oxygen more efficiently. Other researchers identified the gene that encodes EPO and showed that it becomes more active under low-oxygen conditions.
That’s when Gregg Semenza and his collaborators entered the picture. Reasoning that the higher gene activity is controlled by a nearby piece of DNA, they put the EPO gene and some of its surrounding DNA into mice and looked at whether it remained oxygen responsive. By altering the amount of neighboring DNA, Semenza and his colleagues were ultimately able to identify a short, 50 base pair piece of DNA that could confer oxygen-responsive activity to other genes. The lab where his Nobel co-recipient Ratcliffe works published similar results around the same time.
This work also showed that whatever was driving the oxygen-responsive gene activity was present in most cell types. This wasn’t just a mechanism for regulating EPO in the kidneys but, rather, a universal mechanism for sensing oxygen levels.
To identify this mechanism, the researchers performed a clever bit of biochemistry. Scientists regularly separate DNA by running it through gels, where the meshwork of the gel material slows down DNA molecules based on their size. If a protein is sticking to the DNA, it will slow the DNA down even more. The researchers used this slowdown as an assay to identify mixtures of proteins that included ones that could stick to the DNA that regulates EPO. This led the researchers to the sequence of the protein and then, ultimately, to the gene.
Or, in this case, genes. Two proteins seemed to stick to this sequence when oxygen levels were low. One of these, as it turns out, is a general factor that’s active in a variety of cells under all conditions, and it interacts with multiple gene-regulation networks. The other protein, however, was only present when oxygen levels were low. Semenza called it HIF-1α. Its discovery led to the identification of a closely related protein, EPAS1; collectively, the two mediate a suite of oxygen-sensitive activities.
Regulation through destruction
There was something strange about both HIF-1α and EPAS1: while the proteins were only present when oxygen levels were low, their genes were transcribed into RNA all the time. That suggested something else was happening between the RNA being made and the protein being present. Usually, there are only three ways to explain this: destruction of the RNA before it’s translated into protein, failure to translate it into protein, or destruction of the protein after its made. A large group of labs all generated results that indicate the last of these was key here: HIF-1α and EPAS1 are only stable when oxygen levels are low.
That’s where Kaelin enters the story. His group, along with others, had been working on a gene called VHL due to its ability to suppress cancerous growth. A lot of genes had altered activity in cells that lack VHL, and Kaelin and other researchers noted that a number of these genes were also regulated by HIF-1α. Ratcliffe picked up on these results and showed VHL targets HIF-1α for destruction when oxygen is present (it does so by attaching copies of a “destroy me” signal called ubiquitin to HIF-1α).
Why is this step oxygen-sensitive? Researchers found that VHL binds to HIF-1α through a specific sequence of amino acids, but only when oxygen is around. When oxygen is present, this sequence would be chemically modified in a way that enhances the interaction between HIF-1α and VHL. If oxygen is not present, the chemical modification doesn’t take place, and HIF-1α and VHL don’t interact; this allows HIF-1α to survive long enough to alter the activity of the genes it regulates.
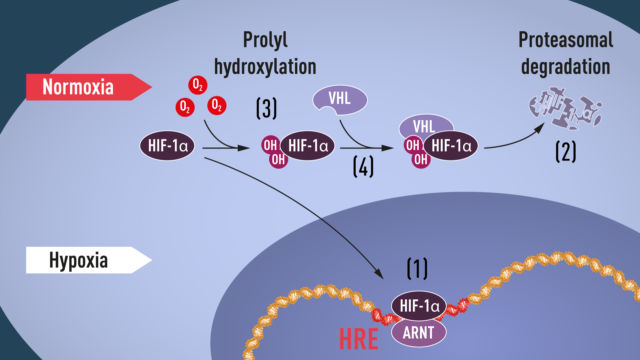
Since this sort of chemical modification had been described for other proteins, it was a relatively simple matter to identify the protein that modifies HIF-1α when oxygen is present.
But that wasn’t the end of the story. Some of the same researchers found a separate region of HIF-1α that was also chemically modified when oxygen was present. In this case, the oxygen-dependent modification prevented interactions between HIF-1α and proteins that connect with the complex that transcribes DNA into RNA. Thus, if any HIF-1α somehow survives digestion, it will not be able to activate any genes near where it sticks to DNA.
Oh yes, the medicine stuff
Technically, the award is in Physiology or Medicine, and the oxygen response is most certainly physiology. But there are also medical applications here. While EPO has been used to treat various forms of anemia, it needs to be refrigerated and injected for use. A number of small molecule drugs that can be taken as pills has been designed to block the oxygen-dependent modifications of HIF-1α, increasing its activity and thus increasing EPO production.
But the oxygen response is tied into a huge variety of biochemical pathways. Cells in solid tumors often end up without a good blood supply and will trigger it as a way to induce new blood-vessel growth. Plus the connections between the oxygen-sensing pathway and the tumor suppression of VHL are still being explored. Oxygen sensing through this pathway is also involved in the wound-healing process and appears to be active following both heart attacks and stroke; these latter two both involve the loss of a blood supply to tissues.
So, while there are already some limited medical applications of what we’ve learned about HIF-1α, it’s fair to say that others will be likely in the future.
We should also note that the Nobel prize created a real challenge by artificially limiting itself to three researchers. The Nobel committee’s scientific background on these discoveries is littered with other names who contributed part of the picture. In a number of cases, the same names appear multiple times. And of course, these names just represent the lab heads; individual projects almost certainly involved at least a half-dozen lab members.
Finally, the dizzying connections that were enabled by this other work—proteins involved in tumor biology and oxygen sensing, chemical modifications known about from outside the cell targeting HIF-1α, and more—highlight how any individual advances in biology are incremental and draw on data from countless other researchers.
https://arstechnica.com/?p=1581271

