
Scientists have long puzzled over the exceptional preservation of certain fossils of Cenozoic-era biota, including plants, fish, amphibians, spiders, and other insects. The secret: The presence of mats comprised of single-celled microalgae (diatoms) created an anaerobic environment for fossilization and chemically reacted with the spiders’ organic polymers to turn them into thin carbon-rich films. The process is similar to a common industrial treatment to preserve rubber, according to a recent paper published in the journal Communications Earth & Environment.
Most fossils are basically mineralized body parts: shells, bones, and teeth. But softer tissues are far more likely to decay than fossilize, including chitinous exoskeletons, skin, and feathers. Soft-tissue organisms tend to be under-represented among fossils, except for unusual deposits (called Fossil-Lagerstätten) that boast rich arrays of such fossils in remarkable preservation.
“Most life doesn’t become a fossil,” said Alison Olcott, a geologist at the University of Kansas. “It’s hard to become a fossil. You have to die under very specific circumstances, and one of the easiest ways to become a fossil is to have hard parts like bones, horns, and teeth. So, our record of soft-body life and terrestrial life, like spiders, is spotty—but we have these periods of exceptional preservation when all circumstances were harmonious for preservation to happen.”
The study by Olcott et al. involved eight fossilized spiders—currently stored in the National Museum of Natural History in Paris— excavated from one such site of exceptional preservation known as the Aix-en-Provence formation, known for its abundance of fossil fish, plants, and arthropods. The samples were found in the “Insect Bed,” which has been a site of interest to scientists since the late 1700s.
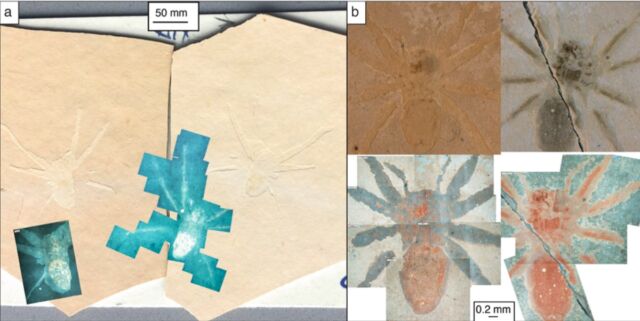
Olcott and co-author Matt Downen (now assistant director of KU’s Center for Undergraduate Research) were examining the fossils when they had the idea to stick them under a fluorescent microscope. Surprisingly, the fossils glowed, prompting the team to investigate further to determine whether this could be a clue to the fossils’ remarkable preservation.
“If you just look at the fossil on the rock, they’re almost indistinguishable from the rock itself, but they glowed a different color under the fluorescent scope,” said Olcott. “So, we started exploring the chemistry and discovered the fossils themselves contain a black polymer made of carbon and sulfur that, under the microscope, looks like the tar you see on the road. We also noticed there were just thousands and thousands and thousands of microalgae all around the fossils and coating the fossils themselves.”
Prior studies suggested the diatom mats would have created an anaerobic environment, thanks to extracellular polymeric substances. This would have slowed down bacterial decay sufficiently to allow the softer organisms to fossilize. But Olcott et al. thought this explanation was insufficient, particularly since it wouldn’t account for why similar bacterial mats—which produce the same polymeric substances—did not help preserve such organisms.
So the team followed up the fluorescent microscopy with scanning electron microscopy (SEM) imaging, which revealed a black polymer on the fossil and two kinds of microalgae. Chemical analysis showed that the microalgae were mostly made up of silica, but the polymer was rich in sulfur. They also used energy-dispersive X-ray spectroscopy and found that the brown material within the fossils was mostly sulfur and carbon.
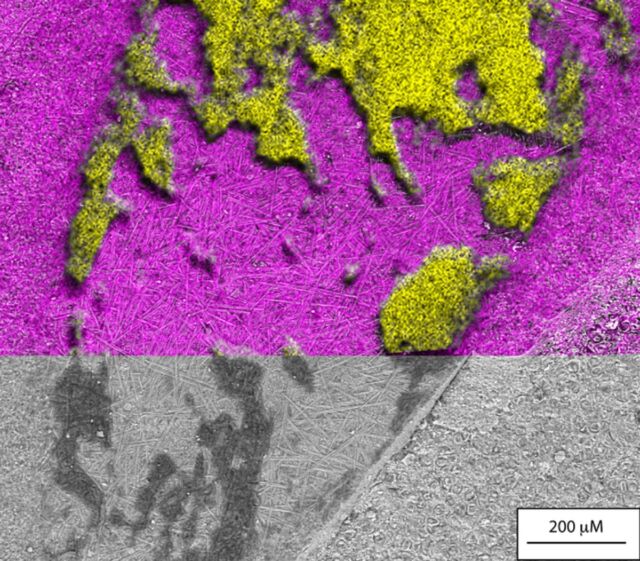
The authors suggest that the combination of the sticky goop produced by the microalgae and the chemistry of the spiders resulted in sulfurization (natural vulcanization)—a chemical reaction that can occur in oxygen-depleted environments. Essentially, the chemical reaction involves sulfate compounds that are transformed into sulfides, cross-linking with the organic molecules (biopolymers), thereby stabilizing and preserving any organic matter. “The resulting polymer is quite resistant to degradation, as the carbon is no longer easily bioavailable and is stable,” the authors wrote. “Studies have shown that this type of naturally vulcanized compound can be preserved in the rock record for millions of years.”
Under this scenario, a spider in the Cenozoic era would expire and become entrapped in a diatom mat. Over time, pieces of that mat would fall to the sediment floor, which contained other diatoms and algae. Spider exoskeletons are made of chitin (composed of long polymers with carbon units), along with proteins and glycoproteins, and those biopolymers are well-suited for sulfurization. Once the spider had been polymerized, the sediments slowly compressed, preserving the spider as a fossil in the rock layer.
Olcott and her colleagues did much of the analysis during the pandemic, which forced many scientists to figure out workarounds while laboratories were shut down. “I had to change how I was doing science,” said Olcott. “I spent a lot of time with these images and these chemical maps and really explored them in a way that probably wouldn’t have happened if all the labs were open and we could have gone in and done more conventional work.”
The team will expand their research to imaging other fossil deposits to discover whether the diatom mats are also linked to preservation more broadly. Per Olcott, around 80 percent of Cenozoic era fossil sites are surrounded by microalgae.
DOI: Communications Earth & Environment, 2022. 10.1038/s43247-022-00424-7 (About DOIs).
https://arstechnica.com/?p=1850369

