Pfizer Inc. PFE, along with partner OPKO Health, Inc. OPK, announced that the FDA has issued a Complete Response Letter (“CRL”) to the biologics license application (“BLA”) for somatrogon for the treatment of growth hormone deficiency (“GHD”) in pediatric patients.
 – Zacks
– Zacks Shares of both Pfizer and OPKO were down in pre-market trading on Monday following the announcement of the news.
Pfizer and OPKO signed the global deal for somatrogon in 2014, per which OPKO is mainly responsible for clinical development while Pfizer will take care of commercializing the product.
Pfizer is currently evaluating the FDA’s comments and is looking to work with the regulatory body to decide the best path forward for somatrogon.
Per the press release, GHD affects one out of approximately 4,000-10,000 children. Somatrogon is a long-acting recombinant human growth hormone that will be administered once weekly, following approval.
Shares of Pfizer have rallied 41.6% in the past year compared with the industry’s increase of 9.7%.
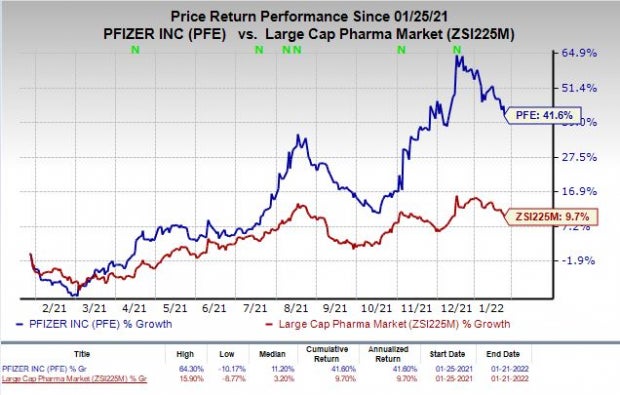 Image Source: Zacks Investment Research
Image Source: Zacks Investment Research
Shares of OPKO have plunged 15.6% in the past year compared with the industry’s decrease of 10.5%.
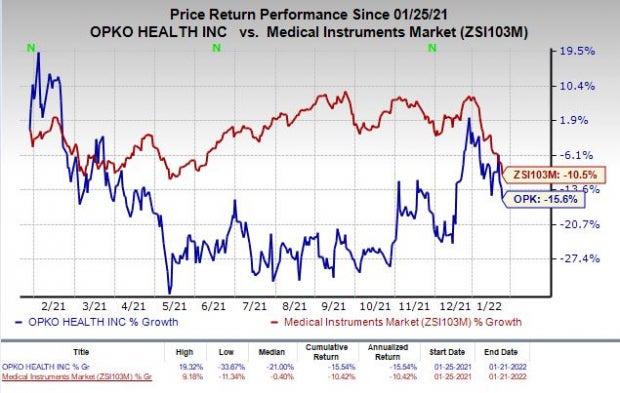 Image Source: Zacks Investment Research
Image Source: Zacks Investment Research
Earlier this month, Japan’s Ministry of Health, Labour and Welfare approved NGENLA (somatrogon) Inj. 24 mg Pens and 60 mg Pens for the indication of short stature resulting from GHD without closed epiphyses (another term for pineal gland).
In January 2021, the FDA accepted the BLA for somatrogon to treat pediatric patients with GHD. A decision from the regulatory body was expected in October 2021.
In September 2021, the review period for the BLA for somatrogon was further extended by three months. A decision from the FDA was then expected in January 2022. The extension of the review period by the FDA is a result of the submission of additional data by Pfizer in addition to the data submitted at the time of the initial BLA filing.
The latest CRL from the FDA is expected to further delay the approval for somatrogon in the United States.
In December 2021, the European Medicines Agency’s Committee for Medicinal Products for Human Use rendered a positive opinion on, and recommended marketing authorization for somatrogon to treat pediatric GHD. A decision from the European Commission (“EC”) is expected shortly.
In January 2022, the EC granted marketing authorization to Ascendis Pharma’s ASND TransCon hGH (Lonapegsomatropin Ascendis Pharma) for treating GHD in children and adolescents aged between three to 18.
In August 2021, Ascendis announced that the FDA approved TransCon hGH (under the trade name of Skytrofa) as a treatment for growth failure due to inadequate secretion of endogenous growth hormone in pediatric patients aged one or above and weighing at least 11.5 kg.
Zacks Rank & Key Pick
Pfizer currently carries a Zacks Rank #1 (Strong Buy) while OPKO currently carries a Zacks Rank #3 (Hold). A top-ranked stock in the drug/biotech sector is Alkermes plc ALKS, which flaunts a Zacks Rank #1 at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Alkermes’ earnings estimates have been revised 1.4% upward for 2022 over the past 60 days. The stock has increased 5% in the past year.
Alkermes’ earnings have surpassed estimates in each of the trailing four quarters.
Zacks’ Top Picks to Cash in on Artificial Intelligence
This world-changing technology is projected to generate $100s of billions by 2025. From self-driving cars to consumer data analysis, people are relying on machines more than we ever have before. Now is the time to capitalize on the 4th Industrial Revolution. Zacks’ urgent special report reveals 6 AI picks investors need to know about today.
See 6 Artificial Intelligence Stocks With Extreme Upside Potential>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE): Free Stock Analysis Report
Alkermes plc (ALKS): Free Stock Analysis Report
OPKO Health, Inc. (OPK): Free Stock Analysis Report
Ascendis Pharma AS (ASND): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
https://www.entrepreneur.com/article/414801

